At present, valve-regulated lead-acid batteries are widely used in electric operating power supplies. Due to the special structure of the valve-regulated lead-acid battery, it is difficult to reliably detect the performance of the battery during operation, and it is difficult to maintain the battery in a targeted manner. It is very urgent. Various battery monitoring systems are also widely used from the high reliability requirements of power system operation. However, different test modes reflect different performances of the battery. Years of research and application have shown that internal resistance detection is one of the most reliable test methods, and different failure modes of the battery reflect different internal resistance. To understand the relationship between the internal resistance of the battery and various failure modes, it is better to better detect and maintain the battery. Reasonable selection and use of the battery and battery monitoring modules in the current DC power system has a great effect on extending the service life of the battery, and is of great significance for obtaining maximum safety and economic benefits.
2 common battery failure modes
For valve-regulated lead-acid batteries, the usual performance deterioration mechanisms are: battery water loss, corrosion of positive plate group, shedding of active properties, passivation caused by deep discharge, and recovery after deep discharge, etc. Performance deterioration:
1, the battery is dehydrated
Loss of water in the lead-acid battery will cause the specific gravity of the electrolyte to increase, causing corrosion of the positive grid of the battery, and reducing the active material of the battery, thereby reducing the capacity of the battery and failing.
In the later stage of valve-regulated lead-acid battery charging, the oxygen released from the positive electrode contacts the negative electrode, reacts, and regenerates water.
O2 + 2Pb→2PbO
PbO + H2SO4→H2O +PbSO4
The negative electrode is in an undercharged state due to the action of oxygen, so that no hydrogen gas is generated. The oxygen of this positive electrode is absorbed by the lead of the negative electrode, and the process of synthesizing water is further developed, that is, the so-called cathode absorption.
In the above cathode absorption process, since the generated water can not overflow under the condition of sealing, the valve-regulated sealed lead-acid battery can be spared from the supplementary water maintenance, which is also the origin of the valve-regulated sealed lead-acid battery called the dimension-free battery. The battery should not escape during the storage period; no charge should escape when the charging voltage is below 2.35V/monomer (25°C); no gas should escape during the discharge. However, when the charging voltage exceeds 2.35 V/cell, it is possible to cause gas to escape. Because at this time, the battery body generates a large amount of gas in a short time and can not be absorbed by the negative electrode. When the pressure exceeds a certain value, it starts to be exhausted through the one-way exhaust valve, and the exhausted gas is filtered by the acid pad to filter out the acid mist. The loss of gas in the battery is also equal to the loss of water. Therefore, the valve-regulated sealed lead-acid battery has a very strict charging voltage requirement and must not be overcharged.
2. Negative plate sulfation
The main active material of the battery negative grid is sponge-like lead, and the following chemical reaction occurs in the negative grid when the battery is charged.
PbSO4 + 2e = Pb + SO4-
Oxidation reaction occurs on the positive electrode:
PbSO4 + 2H2O = PbO2 + 4H+ + SO4- + 2e
The chemical reaction occurring during the discharge process is the reverse reaction of this reaction. When the charge of the valve-regulated sealed lead-acid battery is insufficient, PbSO4 exists on the positive and negative grids of the battery. PbSO4 will lose its activity for a long time. Participation in chemical reactions, this phenomenon is called sulfation of active substances, in order to prevent the formation of sulfation, the battery must always be in a fully charged state, the battery must not be over-discharged.
3. Positive plate corrosion
Due to the water loss of the battery, the specific gravity of the electrolyte is increased, and the acidity of the excessively strong electrolyte increases the corrosion of the positive electrode plate, and the corrosion of the electrode plate must be prevented to prevent the water loss phenomenon of the battery.
4, thermal runaway
Thermal runaway means that the battery has a cumulative enhancement of charging current and battery temperature during constant voltage charging, and gradually damages the battery. The root cause of thermal runaway is that the float voltage is too high.
In general, it is appropriate to set the float charge to 2.23 ~ 2.25V / monomer (25 ° C). If you do not work according to this float range, but use 2.35V / monomer (25 ° C), thermal loss may occur after continuous charging for 4 months; or 2.30V / monomer (25 ° C), continuous charging 6 ~ Thermal runaway may occur in 8 months; if 2.28V/monomer (25°C) is used, severe capacity loss will occur for 12 to 18 months, resulting in thermal runaway. The direct consequence of thermal runaway is that the battery casing is bulged, leaking, and the battery capacity is reduced, eventually failing. 3 Research on internal resistance model of valve-regulated lead-acid battery
Impedance analysis is a common method in electrochemical research and a necessary means for battery performance research and product design [10].
Figure 2-1 is a typical lead-acid battery impedance diagram, which can be seen in the following sections:
1) the inductance part reflected after 100Hz;
2) high frequency resistance RHF, that is, the real part after exceeding 100 Hz;
3) The first small capacitive ring (radius R1) between 0.1 Hz and 100 Hz;
4) The second large capacitive ring (radius R2) below 0.1 Hz.
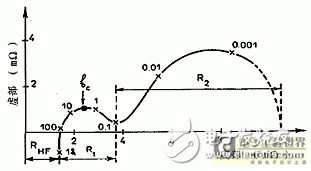
Figure 2-1 Battery impedance spectrum
Fig.2-1 Spectrum of battery impedance
Regarding the battery impedance spectrum, the general explanation is:
a) the inductiveness of the portion exceeding 100 Hz is the effect of the internal geometry of the battery and the connecting components;
b) The ohmic resistance RHF includes the connection resistance, the diaphragm resistance, the electrolyte resistance and the surface resistance of the electrode to the lead sulfate crystal;
c) that the small capacitive ring is related to the porosity of the electrode;
d) The large capacitive ring is dependent on the electrode reaction and its rate is limited by the Pb2+ ion mass transfer rate.
In many research methods [52], the equivalent circuit of Figure 2-2 is used to represent the battery.
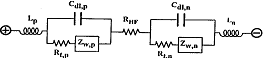
Figure 2-2 Battery impedance equivalent circuit
Fig.2-2 Equivalent circuit of battery impedance
In Figure 2-2, Lp and Ln are positive and negative inductances;
Rt.p and Rt.n are electrode ion migration resistances;
Cdl.p, Cdl.n are plate electric double layer capacitors;
Zw.p and Zw.n are Warburg impedances, which are determined by the diffusion rate of ions in the electrolyte and the porous electrode;
RHF is the aforementioned ohmic resistor.
In the literature [104], the Warburg impedance is expressed as an impedance ZW composed of a series of resistors and capacitors.
![]()
(2-4)
Where λ is the Warburg coefficient, indicating the diffusion mass transfer characteristics of the reactants and products;
Ω——angular frequency
The impedance of the battery includes ohmic resistance and positive and negative impedance:
Zcell = Zp + Zn + RHF (2-5)
The battery impedance is a complex impedance that is related to the test frequency if other conditions are constant.
In actual use, internal resistance or conductance is often used. The internal resistance is the complex impedance mode, and the conductance is the inverse value of the internal resistance. The two are only the difference of the method.
The internal resistance of a typical case refers to the internal resistance at a fixed frequency. For a general VRLA battery, it can be seen from the impedance spectrum of the battery (2-1) that for frequencies above 100 Hz, the impedance value RHF is An approximate straight line parallel to the Y-axis, also known as ohmic internal resistance.
Our company specializes in the production and sales of all kinds of terminals, copper terminals, nose wire ears, cold pressed terminals, copper joints, but also according to customer requirements for customization and production, our raw materials are produced and sold by ourselves, we have their own raw materials processing plant, high purity T2 copper, quality and quantity, come to me to order it!
Copper Connecting Terminals,Cable Lugs Insulated Cord End Terminals,Pvc Insulated Cord End Terminal,Cable Connector Insulated Cord End Terminal
Taixing Longyi Terminals Co.,Ltd. , https://www.longyicopperlugs.com